Background:
The interpretation of clinical data involving chimeric antigen receptor T-cell therapies (CAR-T's) in Large B-Cell Lymphoma (LBCL) requires a thorough understanding of factors that influence efficacy and safety outcomes.
Materials & Methods:
To identify relevant influencing factors of efficacy and safety outcomes, we conducted a systematic literature review for CAR-T cell therapies. Articles were searched on PubMed from 06.2018 till 06.2023. Data for progression free survival (PFS), overall survival (OS), complete response (CR), objective response rate (ORR), duration of response (DOR), and safety (i.e. Immune Effector Cell-Associated Neurotoxicity Syndrome [ICANS], and Cytokine Release Syndrome [CRS]) were extracted in accordance with the PRISMA guidelines.
Results:
A total of 236 articles were identified and narrowed to 44 publications for full text screening. 13 articles were included in the final analysis. The main reasons for exclusion were other indications, missing data, and lack of significance.
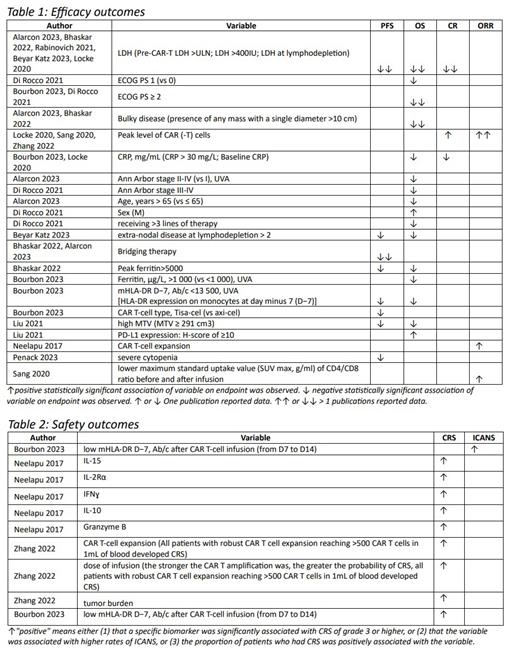
Factors affecting OS that were identified in more than one publication were Eastern Cooperative Oncology Group Performance Status Scale (ECOG PS) ≥ 2, presence of bulky disease, and high lactate dehydrogenase (LDH) values. For PFS it was presence of bridging therapy before CAR-T infusion, and LDH values as well. Table 1 presents the full list of identified variables. For DOR no influencing factor was observed.
Influencing factors for safety outcomes are summarized in table 2. Evidence is less compelling here because all identified variables were supported by one publication only.
Conclusion:
This review provides a set of factors that influences efficacy and safety outcomes of CAR-T therapies in LBCL. It can be used to assess comparability of different treatment arms in randomized controlled studies or to make careful adjustments when performing indirect treatment comparisons in order to better understand the true value of innovative treatments.
Disclosures
Jost:MArS Market Access & Pricing Strategy GmbH: Current Employment; Miltenyi Biomedicine: Consultancy. Riou:Miltenyi Biomedicine: Current Employment. Walzer:MArS Market Access & Pricing Strategy GmbH: Current Employment; Miltenyi Biomedicine: Consultancy. Mahlich:Miltenyi Biomedicine: Current Employment. Borchmann:BMS Germany; MSD Oncology: Honoraria; MSD Oncology; Takeda: Research Funding.